Iron hydroxide oxide CAS #20344-49-4
| Product Name: |
Iron hydroxide oxide |
| Synonyms: |
Ferric oxide hydroxide;Ferrous acid;Iron monohydroxide monooxide;Iron(III) hydroxide, ^y-phase;Iron(III) hydroxide, ^a-phase;Iron(III) hydroxide, gamma-phase;Iron(III)oxide,Ferric hydroxide oxide;Goethite,Ferric hydroxide oxide, Iron(III)oxide |
| CAS: |
20344-49-4 |
| MF: |
FeHO2 |
| MW: |
88.85 |
| EINECS: |
243-746-4 |
| Product Categories: |
Metal and Ceramic Science;Oxides;Inorganics |
| Mol File: |
20344-49-4.mol |
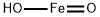 |
|
| Iron hydroxide oxide Chemical Properties |
| Melting point |
135°C |
| density |
3.4-3.9 |
| RTECS |
NO7400000 |
| solubility |
DMSO (Sparingly), Methanol (Slightly), Water (Soluble) |
| form |
crystalline |
| color |
Red-brown |
| Water Solubility |
Soluble in mineral acids. Insoluble in water and ethanol. |
| Merck |
14,4025 |
| Exposure limits |
ACGIH: TWA 1 mg/m3
NIOSH: TWA 1 mg/m3 |
| Stability: |
Stable. |
| CAS DataBase Reference |
20344-49-4(CAS DataBase Reference) |
| NIST Chemistry Reference |
iron(III) oxide hydroxide(20344-49-4) |
| EPA Substance Registry System |
Iron hydroxide oxide (Fe(OH)O) (20344-49-4) |
| Provider |
Language |
| Iron hydroxide oxide |
English |
|
| Iron hydroxide oxide Usage And Synthesis |
| Chemical Properties |
reddish-brown crystalline solid |
| Uses |
In purifying water; as absorbent in chemical processing; as pigment; as catalyst. |
| Uses |
Iron(III) hydroxide, γ-phase is used in purifying water and as an absorbent in chemical processes. It is involved in the preparation of iron oxide-hydroxides nanoparticles, which is used as a very good adsorbent for lead removal from aquatic media. It is also used as a pigment, as a catalyst and in aquarium water treatment as a phosphate binder. Further, it is employed in the paints-lacquers-varnishes industry. |
| Preparation |
A cold solution of 810 g of Fe(NO3)3?9H2O in two liters of water is poured slowly, with vigorous stirring, into an ammonia solution prepared by dissolving 120 g of gaseous NH3 in two liters of water (cooling necessary). The hydroxide which precipitates is amorphous to x-ray analysis. It is washed by stirring at least five times with eight-liter portions of cold water, each portion being decanted as completely as possible. The residual slurry is then stirred with sufficient cone. KOH solution to give a mixture approximately 2N and allowed to stand for 3-4 hours. Finally 100℃ steam is bubbled through for two hours. The precipitate is thereby transformed completely into bright-yellow α-FeO(OH), which shows a crystalline x-ray diffraction pattern.  |
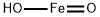










评价
目前还没有评价